IV) Computer Assisted Drug Design
A) Computer Assisted Drug Design
6. Determination of the Acidity of Amidines
Our design concept of nontoxic antitumor antibiotics requires the inclusion of an amidine into the enediyne warhead. In addition, the pKa value of the amidine must be tuned with the help of appropriate substituents in such a way that it can exploit the small acidity difference between normal and tumor cells so that it is no longer active in the normal cell and by this becomes non-toxic.
In our previous work it helped that the pKa values of a large serious of substituted amidines are experimentally known. Nevertheless, for the fine-tuneing of the pKa value of an amidine (needed for a specific enediyne) in dependence of its substituents a more organized strategy is needed.
The determination of pKa values is one of the more ambitious goals of current quantum chemistry. The calculation of proton affinities under the consideration of specific solvation by water molecules is not sufficient. It requires molecular dynamics simulations in a sufficiently large box of water molecules using a suitable QM/MM approach. This we have carried out with success for some small amidines for which an experimental pKa value in aqueous solution is known.
We will focus on the compounds shown in Figure 2 and investigate a) the influence of the dialkynyl groups and b) the influence of the position and the electronic nature of substituent X of the aryl substituent. Although the calculation of pKa values is not an easy task, the structure-acidity relationships already known in the literature and also obtained by the calculations will help to improve results so that pKa values accurate by +/- 0.1 unit will result.
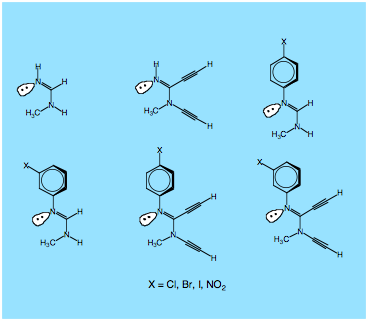
Figure 2. Amidines, for which the pKa value will be determined.
7. Improvement of the delivery system
The biological activity of naturally occurring enediynes depends highly on their docking specificity. For example calicheamicin with a long carbohydrate tail docks much better to the minor groove of DNA than dynemicin, which is reflected by docking energies of 13 and 8 kcal/mol, respectively. It is one goal of experimental work in this field to increase the docking specificity and the docking energy by lengthening of the tail. These experiments are based on tedious synthetic work resulting from the fact that for example the calicheamicin tail contains 17 stereogenic centers, which are essential for the docking properties.
Based on a global representation of the tail, we could show that in the case of calicheamicin the tail possesses a left-handedness, which fits perfectly to the right-handedness of normal DNA helix. This provides a construction principle for any lengthening of the tail. In the case of calicheamicin, the tail consists of the five rings A, B, C, D, and E, where ring E is located on the outside of the minor groove and has more a protecting rather than fixing function. Hence rings A, B, C, and D are responsible for the actual orientation of the tail in the minor groove.
We will extend the tail by stepwise adding to the structure A-B-C-D rings A, B, C, and D and then determine the docking energy in dependence of the tail length. This will show whether certain rings (e.g. the hydrophilic carbohydrate rings A, B, and D) improve the docking properties of the tail more than others (e.g. the hydrophobic benzene ring C), whether a simple duplication of the tail length is more efficient than a stepwise extension (important for experimentalists), and whether the docking specificity of the tail is increased. In connection with the latter question we will test various DNA segments known from clinical tests [1] to be preferred cleavage sites in reactions with naturally occurring enediynes.
Nature can be considered to be the master designer of biochemically active compounds such as the enediynes. 2 billion years of evolution have eliminated all structural possibilities, which did not fulfill the purpose of protecting microorganism against bacteria and viruses. Our work shows how certain structural features are absolutely necessary to orient an enediyne ligand in such a way that it can damage DNA at positions where a repairing of the strands is no longer possible. Also, one can understand why the tail is made up of carbohydrate rings because these facilitate the penetration of the ligand through the cell walls into the cell as well as the docking to DNA. Presently, it is unclear why the carbohydrate tail also contains a benzene ring with a halogen substituent. It is one of the goals of this subproject to clarify the function of benzene ring C.
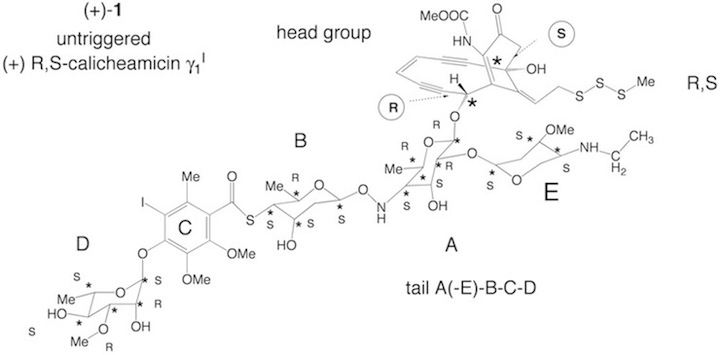
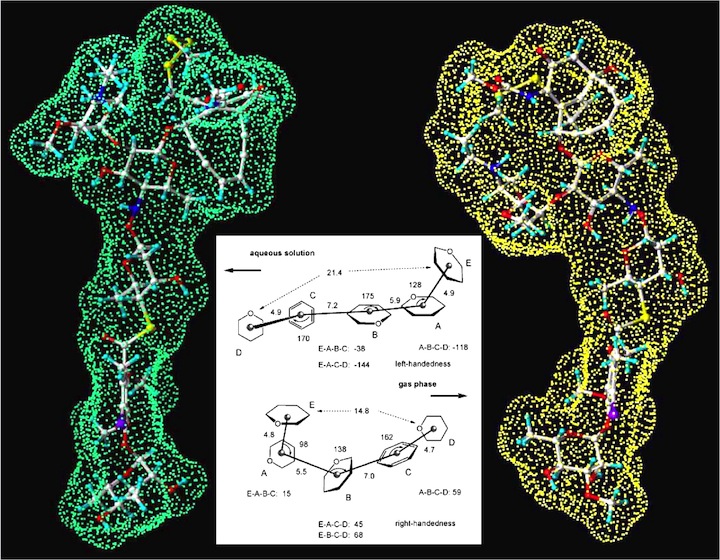
Figure 2. Representation of the carbohydrate tail of calicheamicin according to its solution geometry (left; green Connolly surface) and its gas phase geometry (right; yellow Connolly surface). In the middle the two geometries are simplified by representing each ring by its geometrical center and giving distances and angles between these centers for the solution and the gas phase geometry. Looking from E to D the solution geometry (top) presents a left-handed, the gas phase geometry (bottom) a right-handed screw. Distances in Å, angles in deg.
8. Design of Hybrid Drugs
Despite the structural similarities of the naturally occurring enediynes, enediynes such as calicheamicin, esparamicin or dynemicin dock in different ways into the minor groove, are triggered in different ways, and act in different ways. Clearly, they were designed by nature following different evolution principals. Therefore, it is attractive to combine these different principals to improve the performance of a drug, which may be synthetically easier accessible, but biologically less active and less specific. This leads to hybrid drugs, which are presently discussed in the literature, [2] but which are designed in an ad hoc manner without having detailed insight into the functioning of the parts used for the design of the hybrids.
Our work has made it possible to construct hybrid drugs in a well-organized manner. For example from the docking properties of dynemicin, we identified those positions in the molecule, which could carry a calicheamicin tail to improve the docking selectivity and docking energy. Similarly, there is a possibility of combining the much more efficient triggering device of calicheamicin with the warhead of dynemicin.
The design of hybrid drugs is an ambitious goal because in each case we will specify the ligand geometry by DFT rather than MM calculations since we have found that MM leads to serious errors in describing the naturally occurring enediynes. For example, for calicheamicin the conformation of the carbamate group is wrongly described by all force fields we tested and by this an important part of the triggering is completely missed, which we could demonstrate in our recent DFT work on calicheamicin.
9. Reactions of Enediynes with Proteins
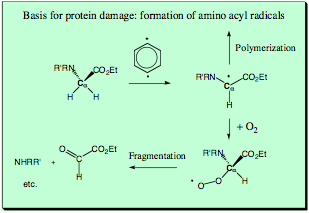
DNA will not be the only target of the enediyne antitumor drugs, which are also capable of cleaving RNA and attacking proteins causing protein agglomeration and apoptosis. Experimental studies involving calicheamicin suggest that its actual toxicity results from damaging the DNA repair enzyme poly(ADP-ribose)polymerase. Esperamicin biradicals attack besides DNA membrane extracts, tubulins and histones where the latter occurs even at low concentrations. Recent investigation show that enediyne-protein interactions lead via transfer of one of the H atoms at a Ca atom to the generation of aminoacyl radicals, which can either dimerize or react with molecular oxygen to form peroxides, which fragment via a transient iminium ion. Electrostatic interaction between enedyine and protein seem to increase the efficiency of the diyl-mediated peptide damage. - Clearly, these observations can be modeled by appropriate QC calculations by reacting a simple dipeptide with the p-didehydrobenzene biradical and with it’s amidine analog.