VII) Ozone and its Reactions
1. Chemical Reactions in the Polluted Atmosphere - A New Source of OH Radicals
A key reaction in atmospheric chemistry is the oxidation of (NO)x to NO2. Nitric oxide (NO)x is produced from the reaction of N2 with O2 in the air during high temperature combustion processes. Fuel combustion in automobiles and industrial plants is the major anthropogenic sources of (NO)x while the largest natural source comes from soil emission. Nitric oxide is oxidized in the atmosphere to NO2, which, if inhaled, hydrolyses in the lungs and leads to dangerous lung-edema. Also, the hydrolysis product nitrous acid can react with secondary amines to form carcinogenic nitrosoamines. NO2 is not only dangerous for human and animal life but also for the flora since it is part of the acid rain. Therefore research in atmospheric chemistry focuses on the oxidation process of (NO)x to NO2, which is known to be triggered by OH radicals.
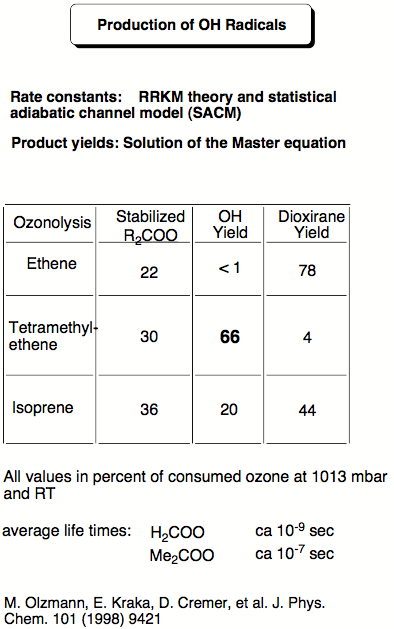
The goal of one of the past research projects was to find out whether the reaction of ozone with alkenes (ozonolysis) can lead to OH radicals and, by this, accelerate the oxidation of (NO)x to NO2 thus deteriorating the quality of urban atmospheres. In urban atmospheres, there is always a large amount of alkenes, which stems from car exhaust, incomplete incineration and industrial smog. The alkenes are oxidized by ozone in the ozonolysis reaction. Since the early 80s, one has speculated that during the ozonolysis of alkenes OH radicals are generated. In a joint project with the research group of R. Schindler at the University of Kiel, we investigated the role of carbonyl oxides, generated as highly reactive intermediates in the ozonolysis, as potential source for OH radicals. R. Schindler and his co-workers had measured up to 40% OH radical production in the ozonolysis of tetramethylethene and, therefore, they assumed that carbonyl oxides are responsible for OH generation. Carbonyl oxides have such a short life-time that all attempts to experimentally investigate their fate during the ozonolysis have failed. The only information on the reactions of carbonyl oxides generated in the ozonolysis is based on ab initio calculations with rather accurate correlation corrected methods.
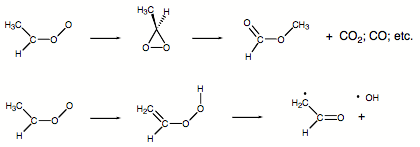
Our ab initio calculations showed that carbonyl oxides predominantly rearrange to dioxiranes which then decompose to esters, CO, CO2, water, and other compounds. However, the ab initio calculations also revealed that for certain methyl substituted carbonyl oxides an intermediate hydroperoxy alkene is formed via H migration. Subsequent cleavage of the OO bond of the hydroperoxy molecule leads to OH and formyl radical production.
Figure 3.1 gives the calculated reaction energies and reaction barriers for the rearrangement of dimethylcarbonyl oxide (# 8 in the Figure, formed in the ozonolysis of tetramethylethene) either to dimethyldioxirane (# 10) or to hydroperoxy propene (# 12). According to ab initio and density functional theory (DFT) calculations, the formation of the hydroperoxide #12 has a lower barrier (# 11, 13.6 kcal/mol) than the isomerization to dioxirane (# 9, 18.7 kcal/mol). Hence, one can expect that OH production (# 13) is preferred in this case. However, the situation is complicated by the fact that carbonyl oxide itself is formed with a high amount of excess energy. Carrying out RRKM calculations in collaboration with Professor Olzmann (Marburg, Germany) we were able to take this into consideration and to quantify the amount OH radicals actually formed. In this way kinetic results of the Schindler group could be fully reproduced and analyzed.
Our results demonstrated that carbonyl oxides are responsible for OH radical production during the ozonolysis of alkenes and this discovery led to a new understanding of OH radical production in the polluted atmosphere. In subsequent work, we investigated the ozonolysis of isoprene. Besides the anthropogenic sources for the alkenes found in urban smog atmospheres, there are also natural sources for alkenes, which cannot be avoided. For example, isoprene is emitted by deciduous trees and other vegetation into the air (together with monoterpenes from conifers 400 000 tons in Northern Europe annually!) Therefore, OH radical formation should be an important factor for the isoprene ozonolysis in a country like Sweden. Our calculations of the ozonolysis of isoprene suggest that up to 20% OH radicals (relative to reacted ozone) are produced in this process.
Since the ozonolysis of an alkene leads to many intermediates that are impossible to investigate experimentally, the ozonolysis represents a problem out of atmospheric chemistry that can only be solved with the help of reliable quantum chemical calculations.
For further reference, see:
- 159Formation of OH Radicals in the Gasphase Ozonolysis of Alkenes: The Unexpected Role of Carbonyl Oxides, R. Gutbrod, R.N. Schindler, E. Kraka and D. Cremer, Chem. Phys. Lett., 252, 221 (1996).
- 176Kinetic and Theoretical Investigation of the Gas-Phase Ozonolysis of Isoprene: Carbonyl oxides as an Important Source for OH Radicals in the Atmosphere, R. Gutbrod, E. Kraka, R.N. Schindler, and D. Cremer, J. Am. Chem. Soc., 119, 7330 (1997).
- 185Energetics, Kinetics, and Product Distribution in Reactions of Ozone with Ethene and 2,3-Dimethyl-2-butene, M. Olzmann, E. Kraka, D. Cremer, R. Gutbrod, and S. Andersson, J. Phys. Chem. A, 101, 9421 (1998).
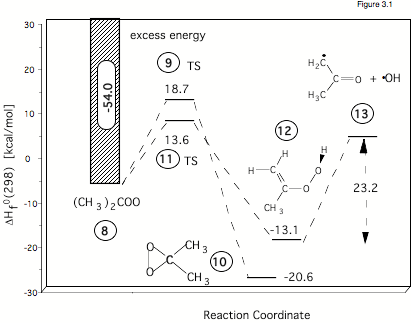
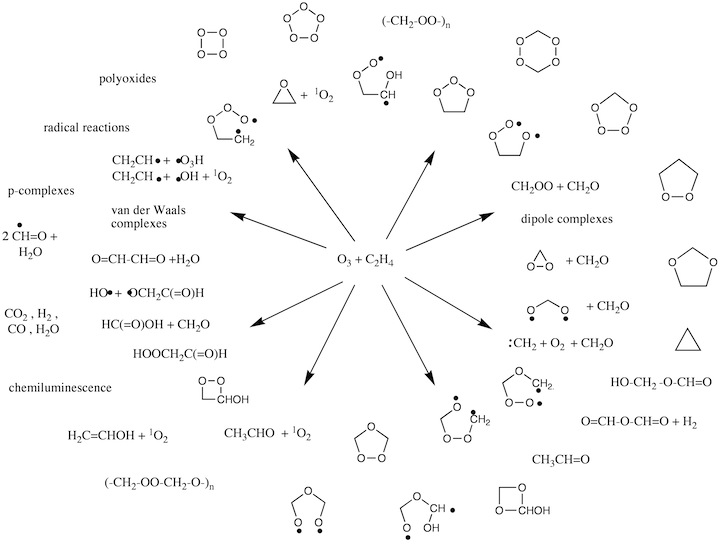
Figure 3.2. Possible intermediates and reaction products generated in the ozonolysis of an alkene.
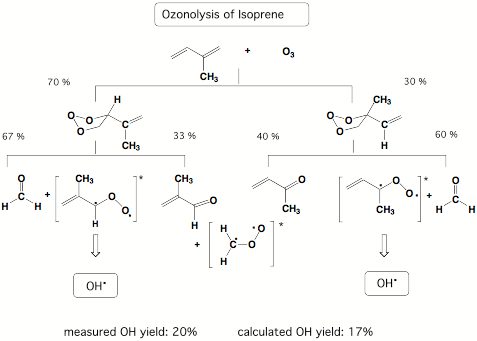
Figure 3.3. Statistical yield analysis for the ozonolysis of isoprene based on RRKM calculations.
2. The Mechanism of the Ozone-Alkyne Reaction
Despite the fact that the ozonolysis of alkynes is known for more than a century, relative little is known about the detailed mechanism of the alkyne ozonation. This has to do with both the complexity of the reaction mechanism and the experimental difficulties of handling potentially explosive intermediates. Nevertheless, the reaction was and still is synthetically used at reduced temperatures to determine the position of triple bonds of larger alkyne molecules, to selectively oxidize enyne molecules or to produce a-diketones and a-ketoesters from alkynes and acetylenic ethers. Furthermore, the alkyne ozonolysis is known to produce epoxidization agents that are considerably stronger than peracids, but so far this has not been exploited in chemical synthesis due of the fact that their structure and other chemical properties are unknown.
It is highly desirable to exploit the synthetic potential of the alkyne ozonolysis in particular as the reaction probably plays also an important role in areas not necessarily related to synthesis: 1) Wastewater and industrial sewage is often purified or treated with ozone and, in this connection, it is desirable to understand the ozone-alkyne reaction in detail. 2) In the atmosphere, the alkyne-ozonolysis is too slow to compete with OH radical-alkyne reactions, but can take place in the absence of OH radicals. 3) It is well-known that that an increase of the ozone concentration in industrial or urban areas leads to the ozonolysis of terpenes emitted from bushes and trees. Similarly, ozone can attack volatile natural products containing CC triple bonds and, by this, generate strongly epoxidizing agents in the atmosphere.
There has been a dispute over years whether the Criegee mechanism applies to both solution phases (where it has been verified) and the gas phase where alternative mechanisms such as the O'Neil-Blumstein mechanism and other radical-driven mechanisms have been discussed. An investigation of the alkyne-ozonolysis is directly relevant for these questions because conclusions drawn for the ethene-ozonolysis can directly be probed in the case of the ozone-acetylene reaction. Acetylene has two H atoms less than ethene and, therefore, the ozonolysis mechanism of Criegee should become simpler. Also it should be easier from a theoretical point of view to test alternatives to the Criegee mechanism, which involve radicals or biradicals.
Hence, the aim of our alkyne-ozonolysis project was threefold. First, we used quantum chemical methods to elucidate the mechanism of the acetylene ozonolysis in detail and to draw general conclusions for the ozonation of alkynes. In this way, we wanted to improve the predictability of product distributions and, consequently, the applicability of the alkyne ozonolysis in synthesis. Secondly, we established the relevance of the acetylene-ozonolysis for the mechanism of the alkene ozonolysis by comparing the two reactions especially with regard to their gas phase and solution phase mechanisms. Finally, we suggested specific experiments that could lead to a confirmation of the results found in our investigation.
The project required DFT and CASSCF calculations besides CCSD(T) and BD(T) calculations.
Molecular energy, geometry, and vibrational frequencies were determined for the systems O3-propene, O3-butene (cis and trans) as well as O3-isoprene. In addition, we determined with the help of statistical calculations the amount of OH radicals obtained in a given ozonolysis system.
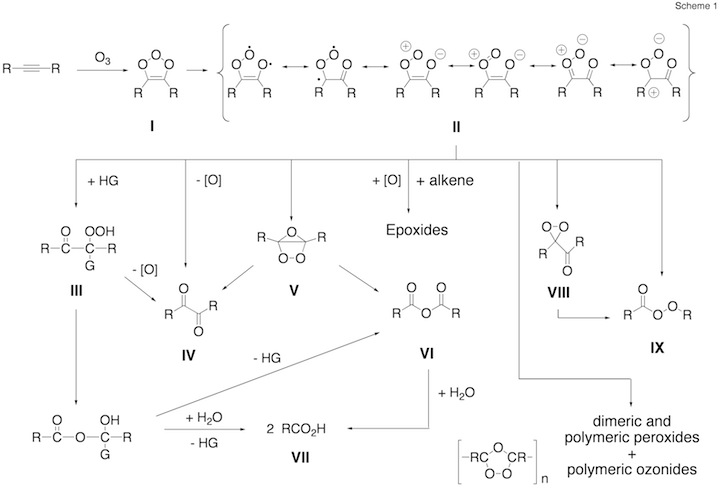
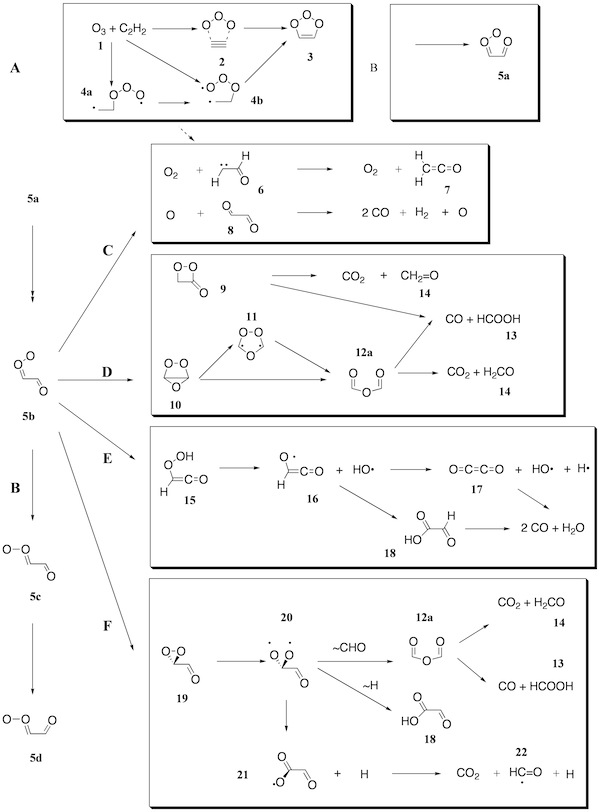
Figure 1. Mechanism of the ozonolysis of acetylene. The various steps of the reaction are coined A (trioxolene formation), B (carbonyl oxide formation), C (oxygen loss), D (cyclization), E (OH production), and F (dioxirane formation).
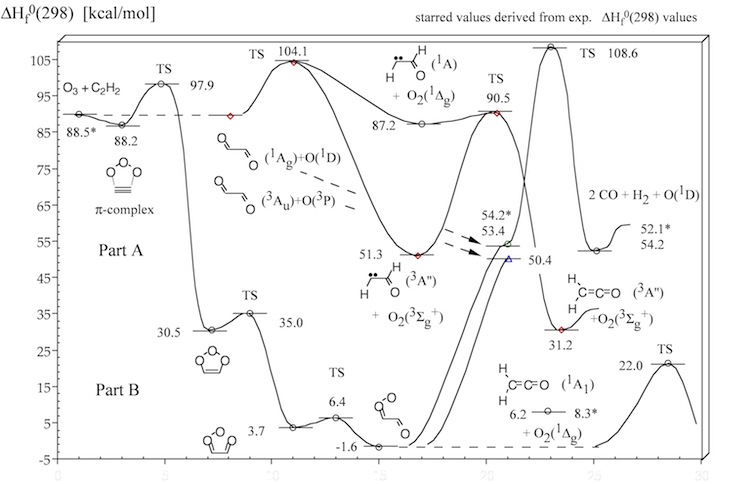
Figure 2. Energy diagram of steps A , B and C of the acetylene ozonolysis (compare with Figure 1). Calculated heats of formation ΔHf°(298) (in kcal/mol) are given for each structure. Experimental ΔHf°(298) values are starred. CCSD(T) and CASPT2 calculations.
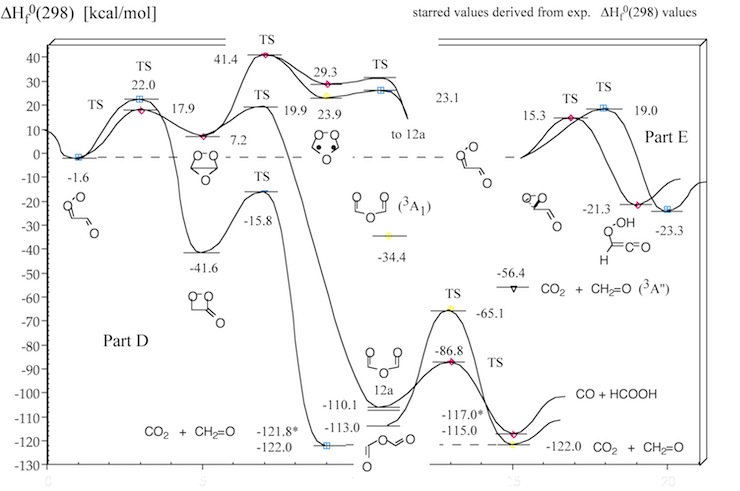
Figure 3. Energy diagram of step D of the acetylene ozonolysis (compare with Figure 1). Calculated heats of formation ΔHf°(298) (in kcal/mol) are given for each structure. Experimental ΔHf°(298) values are starred. CCSD(T) and CASPT2 calculations.
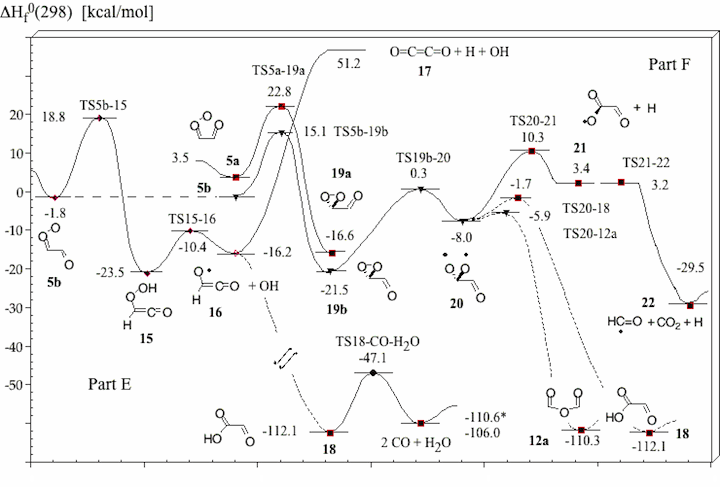
Figure 4. Energy diagram of steps E and F of the acetylene ozonolysis (compare with Figure 1). Calculated heats of formation ΔHf°(298) (in kcal/mol) are given for each structure. Experimental ΔHf°(298) values are starred. TS21-22 is a TS on the PES, but vanishes when ZPE correction is added.
